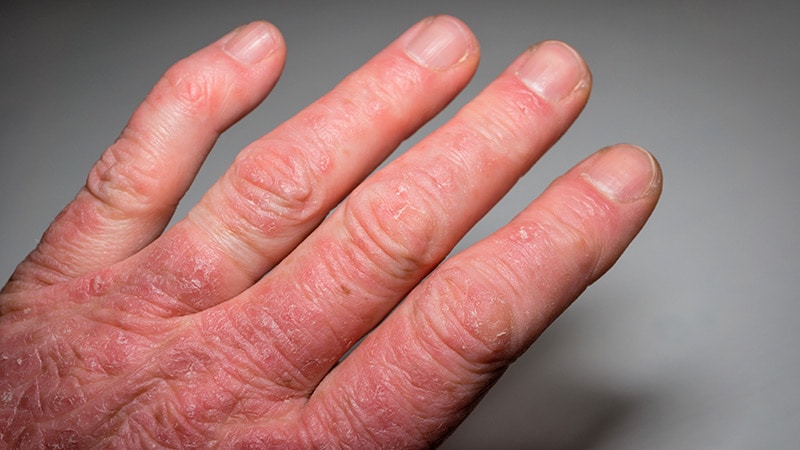
ICIs Associated With Twofold Increased Psoriasis Risk
TOPLINE:
Patients with cancer who receive immune checkpoint inhibitors (ICIs) have more than twice the risk for developing psoriasis compared with those who receive other treatments.
METHODOLOGY:
- Researchers conducted a nationwide cohort study using data from the Taiwan National Health Insurance database and the Taiwan Cancer Registry.
- A total of 135,230 patients with clinical stage III or IV cancer (mean age, 62.94 years; 45.1% women) who received antineoplastic medications between January 2019 and June 2021 were included in the study.
- Patients were classified as ICI users (n=3188) and those who received chemotherapy or targeted therapies were classified as non-ICI users (n=132,042).
- The primary outcome was the incidence of psoriasis during the follow-up period. The mean follow-up duration was 1.46 years.
TAKEAWAY:
- A total of 295 patients (0.2%) were diagnosed with psoriasis. ICI users experienced a higher incidence of psoriasis than non-ICI users (5.76 vs 1.44 cases per 1000 person-years).
- Those treated with ICIs had more than a threefold increase in the risk for psoriasis after adjusting for demographic factors and comorbidities (hazard ratio [HR], 3.31; P <.001) and more than a twofold risk after further adjusting for death as a competing risk (HR, 2.43; P <.001).
- Patients treated with ICIs also showed a higher risk for psoriasis compared than those treated with chemotherapy (HR, 2.69; P <.001), monoclonal antibodies (HR, 2.31; P <.05), and protease inhibitors (HR, 2.34; P <.05) after adjustments.
IN PRACTICE:
“This nationwide cohort study demonstrated that patients with cancer receiving ICIs had a higher risk of developing psoriasis compared to those receiving chemotherapy or targeted therapy. Although this adverse effect is relatively uncommon, it is important for medical professionals, clinicians, and caregivers to be aware of this potential risk to improve skin health and ensure optimal cancer care,” the authors wrote.
SOURCE:
The study was led by Sheng-Yin To, RPh, National Defense Medical Center, Taipei, Taiwan. It was published online on November 6 in JAMA Dermatology.
LIMITATIONS:
The study lacked data on lifestyle and genetic factors, body mass index, and environmental exposures, which may have influenced the incidence of psoriasis. Additionally, the claims database did not provide information on the Psoriasis Area and Severity Index, preventing the assessment of psoriasis severity. The results may be applicable to patients only using programmed cell death 1 (PD-1) and PD-1 ligand-1 inhibitors, as the Taiwan National Health Insurance does not cover cytotoxic T-lymphocyte–associated protein 4 inhibitors.
DISCLOSURES:
The study was supported by the Ministry of Science and Technology of Taiwan and the Tri-Service General Hospital of Taiwan. One author reported receiving research funding from IQVIA outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.